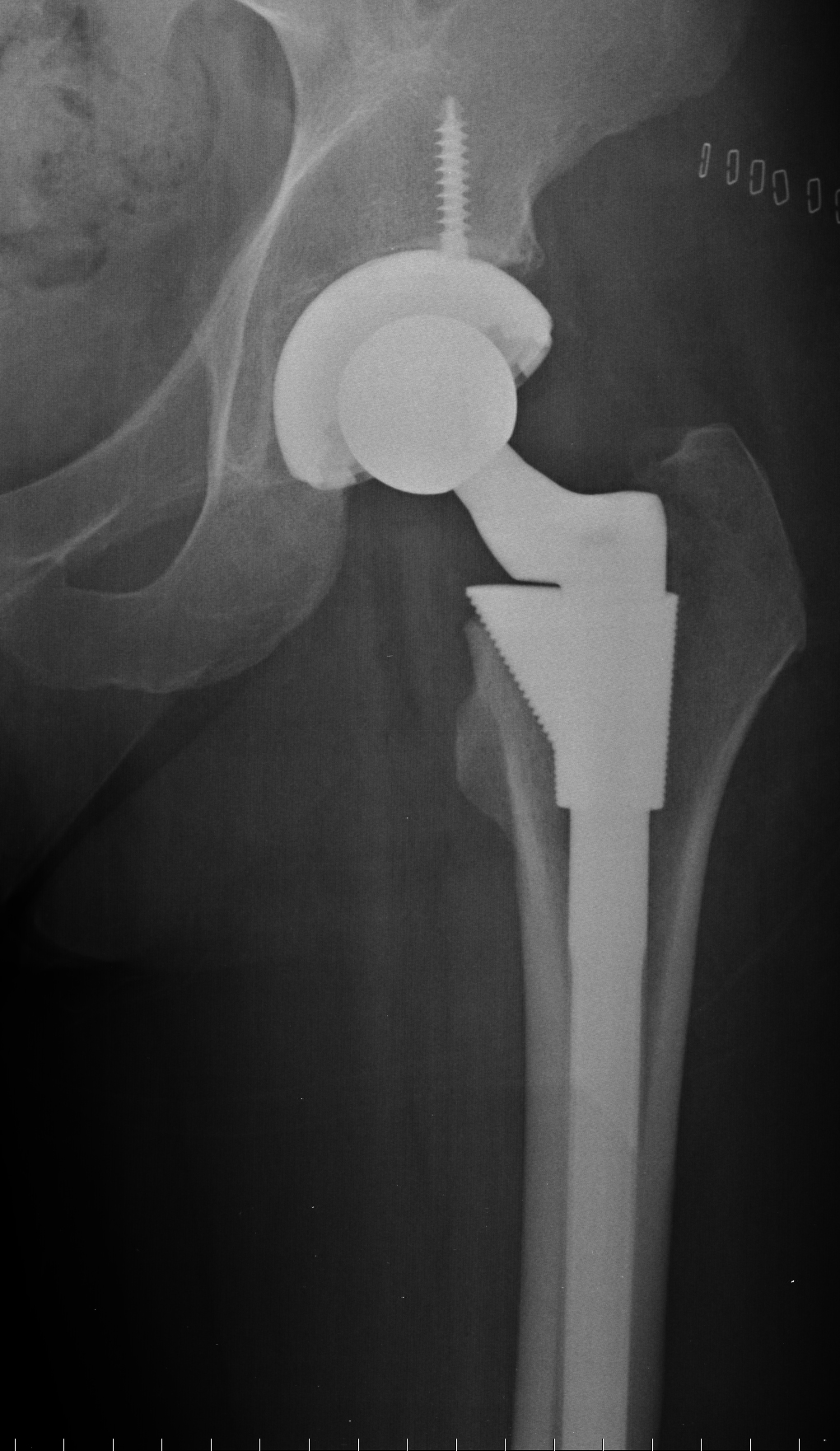
DePuy and Stryker are far from the only medical device manufacturers under fire for producing and promoting defective metal-on-metal hip replacement products. Biomet’s M2A-Magnum hip replacement is now the target of increasing litigation claiming the same disastrous consequences that have occurred under DePuy and Stryker systems. According to the Jere Beasley Report, Biomet marketed this product as having “set the standard for performance and design in hip systems” and as “an ultra-high performance metal-on-metal articulation.” Biomet also asserted that the M2A is superior to other hip replacement products because it is subject to lower wear, excellent stability, better range of motion, and superior joint mechanic restoration.
Metal-on-metal hip replacements may actually be much more risky than the traditional polyethylene hip replacement device. Even though metal-on-metal hip replacements are said to last much longer than their polyethylene counterparts, they are prone to an early failure rate that would likely shock most patients. In addition to the risk for early failure, the health consequences of a metal-on-metal hip replacement can be extremely dangerous. Due to the grinding of metal components, metallic debris may be released into the body and cause serious complications. The problems caused by such grinding include the destruction of surrounding tissue and bone, pseudotumors, and metal blood poisoning. Metal blood poisoning is an especially severe effect of the increase of metal ions in the blood and can even lead to death. Generally, once a hip replacement fails, the patient must undergo at least one revision surgery that can be very complex, painful, and may sustain little hope to re-establish pre-surgery quality of life. Ultimately, the proposed advantages of metal-on-metal hip replacements may be inadequate to outweigh the hazards of such extreme complications.
In one recent West Virginia lawsuit, the plaintiff has argued that Biomet was aware of the risks and failure rates of their product without releasing this information to the public. The West Virginia Record reports that at the time of the plaintiff’s original surgery in 2008, there had already been more than 100 reports of adverse events associated with the M2a Magnum filed with the FDA. It is thus argued that Biomet knew that the product was defective. If Biomet was aware of their product’s severe risks and continued to promote the product as safe, effective, and superior to other hip replacement systems, Biomet could certainly be found guilty of misrepresentation.
Don’t let yourself become a victim of metal-on-metal hip replacement complications. When information is withheld from surgeons, doctors, and patients, it can be difficult to be sure that the procedure you undergo poses no serious health risks. If you believe that you have been misled by the Biomet M2A system, or another defective hip replacement device, it is important to know your rights.
—–
At Borchardt Law Firm, we wish for no family to ever experience incapacitating tragedies due to defective medical devices. Our firm has the experience and the drive necessary to continue to strive for the improved protection of future generations of Texans. If you or a loved one has ever suffered from a related misfortune and feel you were not properly warned about the potential risks, don’t hesitate to contact a lawyer to discuss any legal compensation you might be entitled to. Borchardt Law Firm represent clients over many areas in Texas; feel free to give us a call.
Toll Free: 866.832.9300
Phone: 817.332.9300
Fax: 817.332.9301
firm@attorneysmb.com
801 Cherry St #1005
Fort Worth, Texas 76102
For easy access to blog updates, follow us on Twitter: @AttorneysMB
 Fort Worth Injury Lawyer Blog
Fort Worth Injury Lawyer Blog

