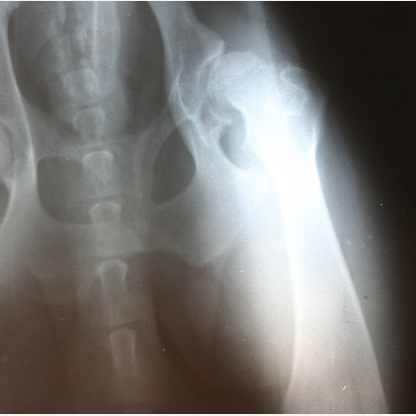
The Stryker Rejuvenate hip implant is a defective medical device that suffers from similar flaws as the DePuy hip replacement product. Elements of this implant contain metal-on-metal qualities that may lead to very serious complications. Many adverse reactions to the product have been reported, internal and external product testing has confirmed the potential dangers of this product, and there are currently lawsuits filed against the manufacturer.
The friction of its metal parts can lead to, according to Stryker, “…a potential for fretting and corrosion at the modular neck junction which may lead to adverse local tissue reactions.” Such reactions include unbearable pain, swelling, and the development of metallosis. Metallosis is the buildup of metal debris in the body caused by the grinding of metal parts. Effects of metallosis include dislocation, bone deterioration, and metal toxicity. These products suffer from a very high early failure rate as well, working directly against their original marketing as a more long-lasting product for younger patients. Often patients implanted with defective devices must consequently undergo extremely costly and painful revision surgeries to correct the trouble.
Additionally, after reports of trouble with Stryker hip implants began to mount, the FDA launched an investigation into one of its manufacturing facilities. The disappointing findings included inadequate quality control measures and even the presence of the Staphylococcus bacteria. In 2007, the FDA warned Stryker to rectify these problems immediately or face very detrimental consequences. These deficiencies are independent of the serious structural problems that plague the two recalled implants.
Reuters reports that Stryker recommends clinical follow-up for all patients affected by the July 2012 recall of Rejuvenate and ABG II modular hip stems. Such clinical follow-up should include blood and imaging tests even if the patient is not experiencing pain, swelling or other symptoms that might suggest hip implant failure. Stryker contends that they have received reports of patients with no or mild symptoms who still found high levels of metal ions in their bloodstream and evidence of local tissue reactions.
Stryker stated that the hip implant recall could ultimately cost between $190 million and $390 million in order to cover patient testing and treatment, new surgeries, lawsuits and insurance payments.
If you have suffered from complications associated with the Stryker Rejuvenate product, don’t hesitate to contact a lawyer and find out what justice you may be entitled to for your physical, mental, or financial distress.
—–
 Fort Worth Injury Lawyer Blog
Fort Worth Injury Lawyer Blog